Adverse Event reporting information can be found in footer
Request a Meeting

View PENTASA formulations to find out more
Paediatric Formulations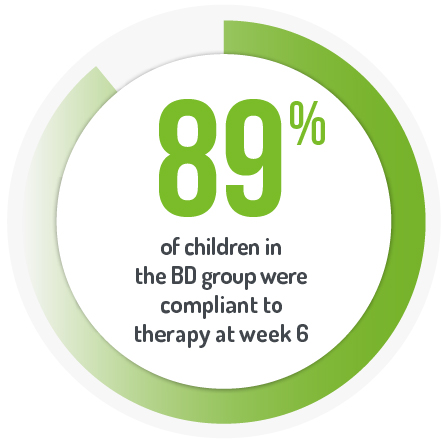
Primary endpoint was mean PUCAI score between the two arms (OD and BD dosing) at week 6. At week 6, there was no difference in median PUCAI score between the OD and BD groups (p=0.48).*
1 g PENTASA sachet
Study Summary
Results from a multi-centre, investigator-initiated, randomised controlled, investigator- blinded induction of remission trial1
| Patient Numbers | Weight | Dose Amount | Dose Frequency |
|---|---|---|---|
| 40 | 15-<20kg | 1,000 mg (500 mg, 500 mg) | Twice-daily |
| 20-<30kg | 1,500 mg (1,000 mg, 500 mg) | ||
| 30-<40kg | 2,000 mg (1,000 mg, 1,000 mg) | ||
| ≥40kg | 3,000 mg (1,500 mg, 1,500 mg) |
Key inclusion criteria
Key exclusion criteria
Safety Results
– Disease exacerbation (3 total)
– Headache (2 total)
– Fever (2 total)
– Nausea (2 total)
Job Code: UK-PA-2400044 - Date of preparation: November 2024