A PERSONALISED APPROACH TO IVF




Adverse Event reporting information can be found in footer
Request a Meeting
Systematic review with one-stage individual participant data (IPD) meta-analysis of randomised, controlled trials.1
To undertake a one-stage meta-analysis of IPD from randomised, controlled trials comparing individualised dosing of REKOVELLE® (follitropin delta) vs other forms of follitropin (alfa and beta) for live-birth rates (LBR) and safety parameters in women undergoing ovarian stimulation for in vitro fertilisation (IVF) treatment.
A systematic review identified eligible phase 3 trials between January 2000 and February 2023. The IPD meta-analysis included women undergoing ovarian stimulation for IVF treatment. All IVF procedures were conducted according to routine clinical practice, including undertaking fresh embryo transfers.
Overall, 2,685 women underwent 2,682 cycles across 3 different trials between October 2013 and May 2020, with live birth follow up until 1st February 2023.
Table 1: Results Overview
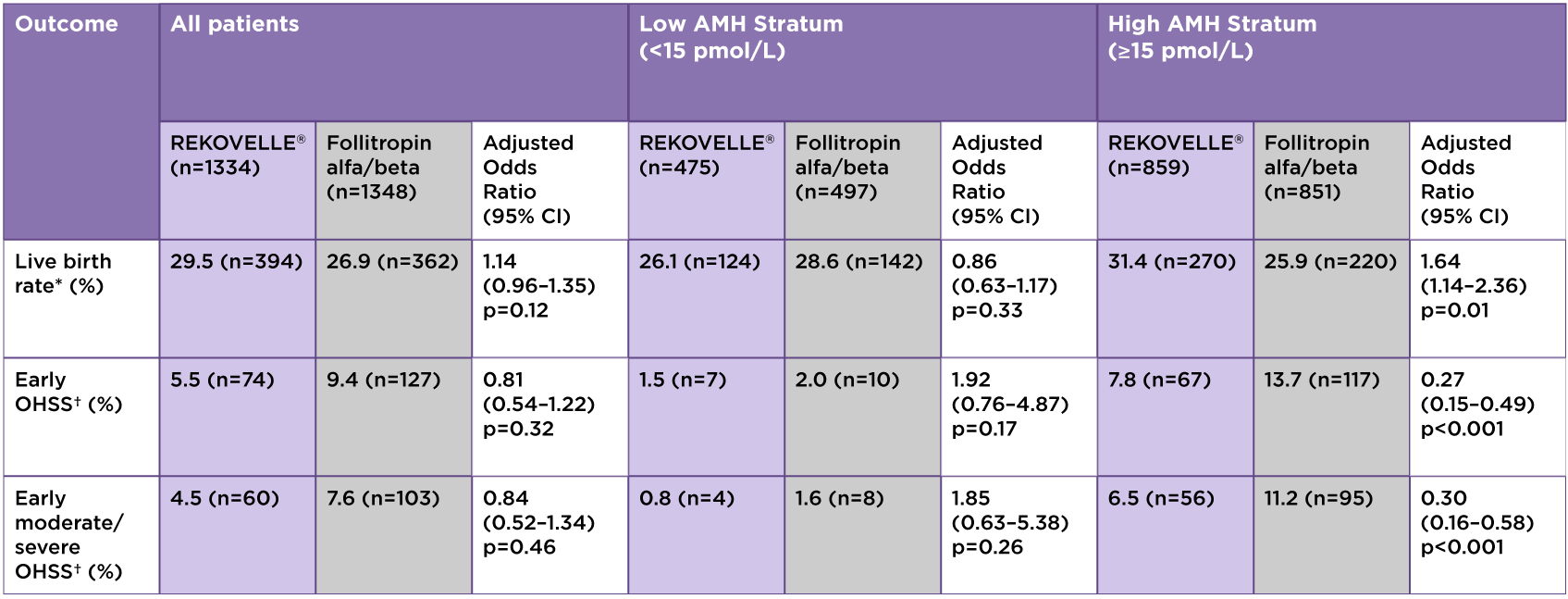
*Primary endpoint; †Or the need for preventative measures.
Adapted from Nelson SM. et al. 2024
KEY PRIMARY OUTCOMES – LBR per cycle started
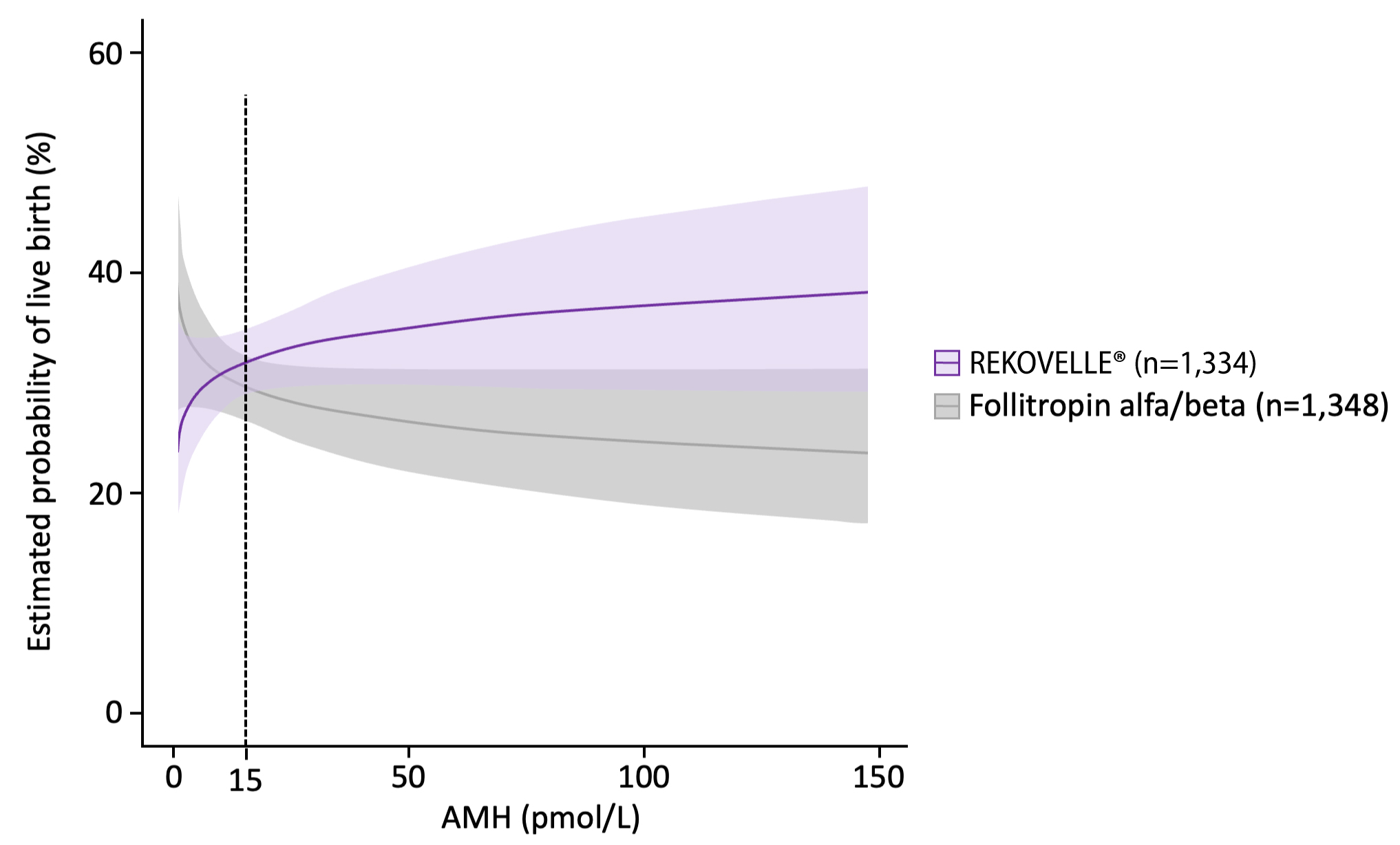
Adjusted OR for patients with AMH <15 pmol/L: 0.86 (95% CI: 0.63–1.17), p=0.33
Adjusted OR for patients with AMH ≥15 pmol/L: 1.64 (95% CI: 1.14–2.36), p=0.01
Adapted from Nelson SM. et al. 2024
KEY SECONDARY OUTCOMES
Figures adapted from Nelson SM. et al. 2024
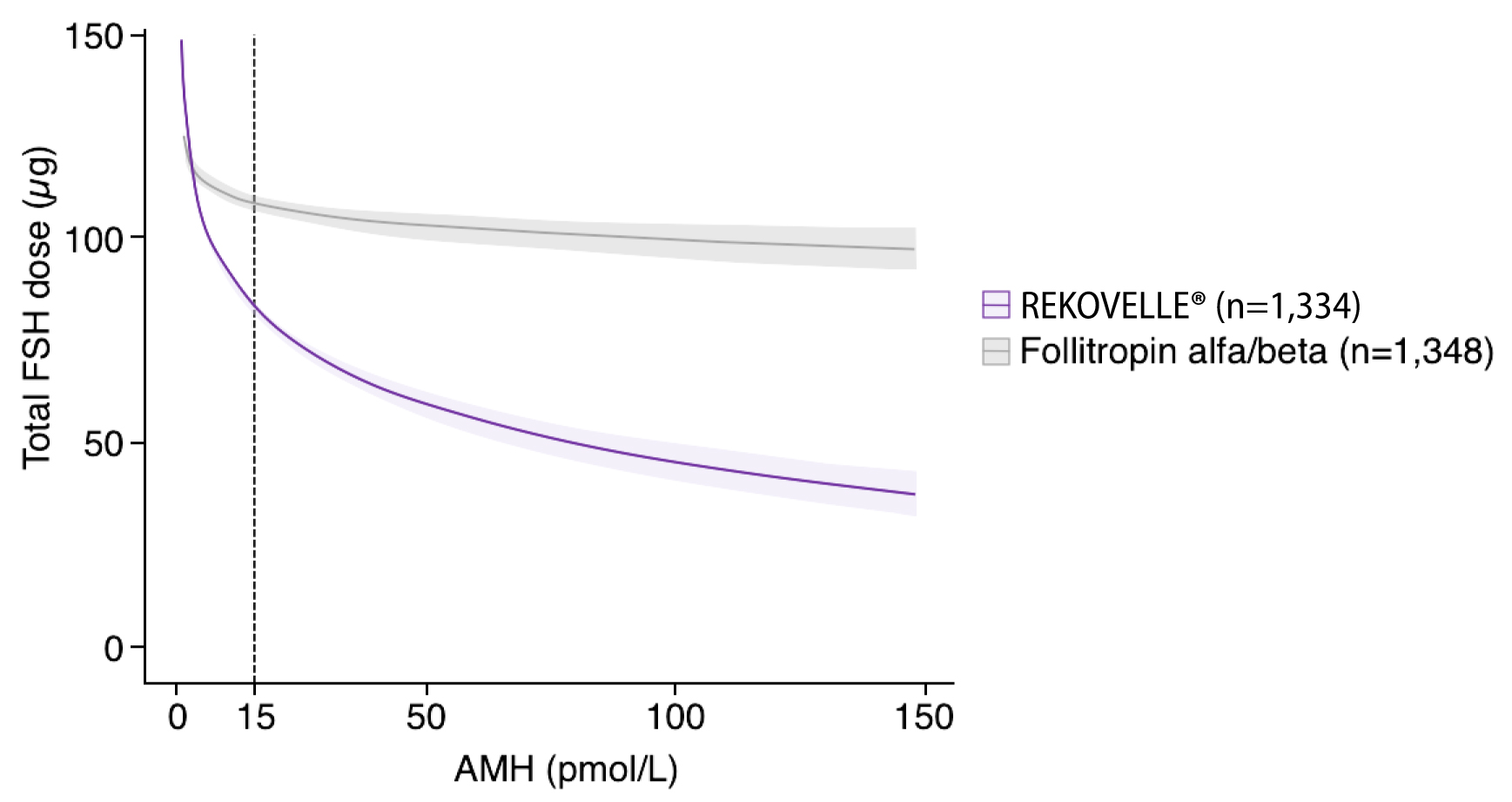
Adjusted mean difference for patients with AMH <15 pmol/L: -11.10 (95% CI: -15.21– -7.00), p<0.001
Adjusted mean difference for patients with AMH ≥15 pmol/L: -48.76 (95% CI: -53.70– -43.81), p<0.001
Adapted from Nelson SM. et al. 2024
Individualised dosing of REKOVELLE® (using AMH level and weight-based algorithm) for ovarian stimulation was associated with increased LBR and reduced risk of early OHSS (and/or the need for preventative interventions) and early moderate or severe OHSS (and/or the need for preventative interventions) in women with elevated AMH levels compared with conventional licensed dosing of follitropin alfa or beta (where the dosage is not adjusted to individual patient characteristics).
Job Code: UK-REK-2400019 - Date of preparation: October 2024