
Consistent colon coverage for your patients 1-5
Click on one of the links below
SUMMARY
PENTASA®, the 5-ASA of choice for your mild to moderate UC patients1-7
PENTASA® is the unique time-dependent formulation which releases 5-ASA regardless of pH1-5
- ensuring consistent proximal to distal colon coverage
- removing the uncertainty of fluctuating intestinal pH levels
Optimised PENTASA® Treatment:
- achieves remission within 2 weeks and maintains remission for 12 months8,9*
- has proven efficacy in moderate and left-sided UC patients8,10,11*
- achieves and maintains high rates of mucosal healing8,11*
- offers once daily formulations and high adherence3-5,12
* Included OD PENTASA® enema for the first 4 weeks
THE ONLY TIME DEPENDENT 5-ASA 1-5,13-27
Optimising mesalazine delivery to the whole colon is important in treating patients with mild to moderate UC
All other 5-ASAs
pH-dependent formulations release 5-ASA where the pH is >6 but can differ in extent of delivery 13-27
For example, Salofalk®’s absorption is highest in proximal gut regions and lowest in distal gut areas 13-19
pH as low as <2.3 has been reported in the colon, suggesting that pH-dependent formulations may not be able to disperse in some patients 10,28
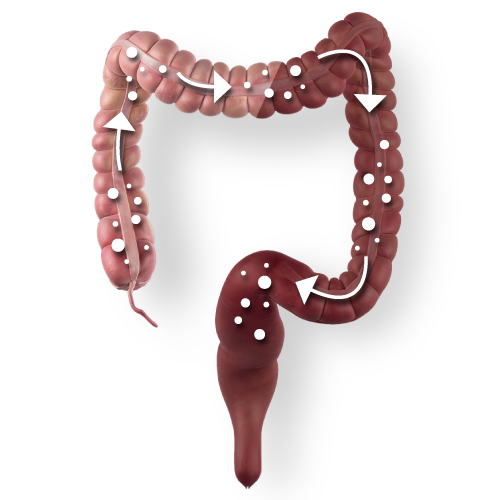
PENTASA®
The only time-dependent formulation which releases 5-ASA regardless of intestinal pH 1-5,13-27
Time-dependent release ensures consistent delivery throughout the entire colon 1-5
80% delivered to the large colon, ensuring efficacy in UC, including left-sided disease 29
ALWAYS SIMPLE ONCE-DAILY FORMULATIONS 1-7

The only 5-ASA sachet licensed to be taken with yoghurt 3-5,16-19
PENTASA® sachets can also be taken with water, orange juice, and with or without food. 3-5
LEFT-SIDED DISEASE
80% of patients with UC have left sided disease 30, and with PENTASA®’s time-dependent release you can achieve consistent delivery of mesalazine throughout the entire colon, ensuring efficacy in LSD. 29
Achieving remission* in 8 weeks 11
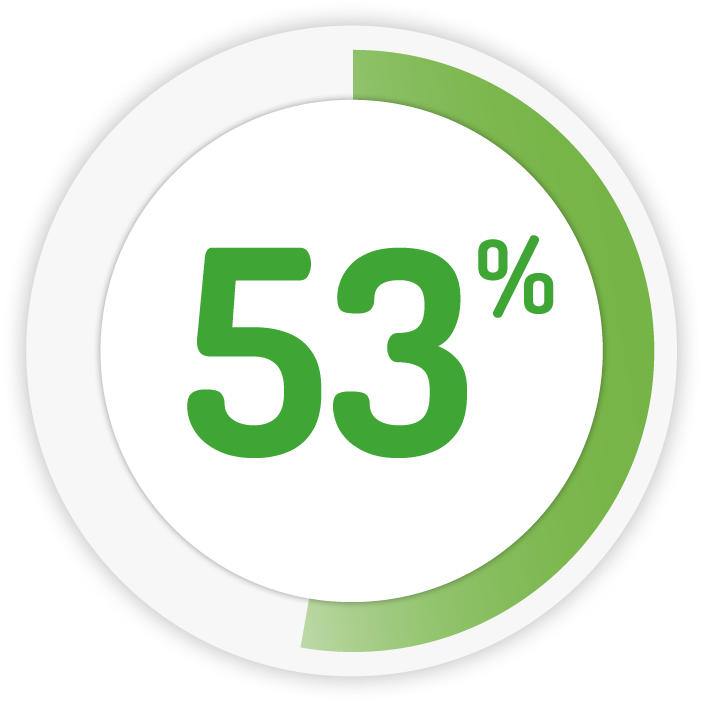

4g daily for 8 weeks

1g OD for 4 weeks
Maintaining remission* to 12 Months 10
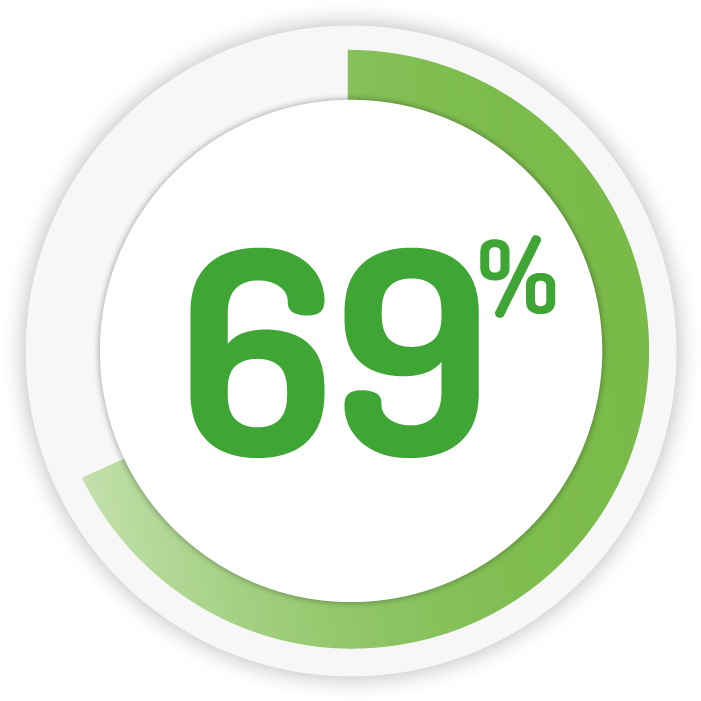

2g OD for 12 months
* Remission: Patients (%) achieving UC-DAI ≤ 1 , Images of medication not shown to scale
MUCOSAL HEALING
British Society of Gastroenterology (BSG) consensus guidelines on managing IBD in adults states that symptomatic remission, with mucosal healing, should be the target of medical therapy in UC 31. Mucosal healing is an important treatment goal and has been correlated with long-term disease remission rates, reduced risk of colorectal cancer, and improved patient quality of life. 11,31
Mucosal Healing Scores* at 8 weeks 11
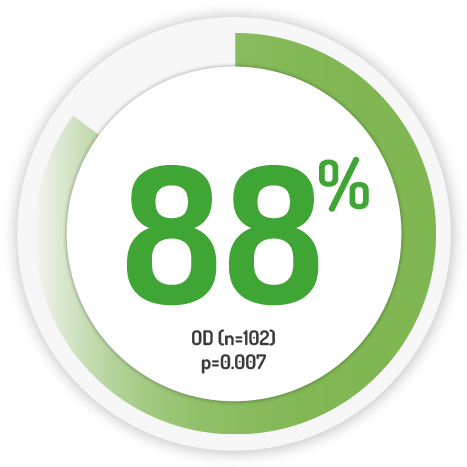

4g daily for 8 weeks

1g OD for 4 weeks
Mucosal Healing Scores* after 12 months 8
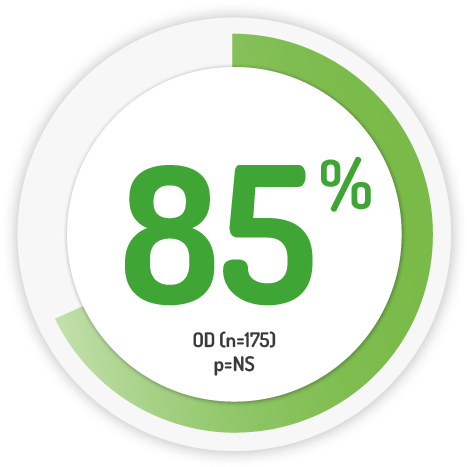

2g OD for 12 months
* Mucosal Healing: UC-DAI endoscopic sub-score of ≤ 1, Images of medication not shown to scale
SPEED OF ACTION - EFFECTIVE IN 2 WEEKS 9
Faster symptom resolution in mild to moderate UC with mesalazine can improve quality of life, and increase treatment adherence. 9
Achieving improvement* in 2 weeks 9
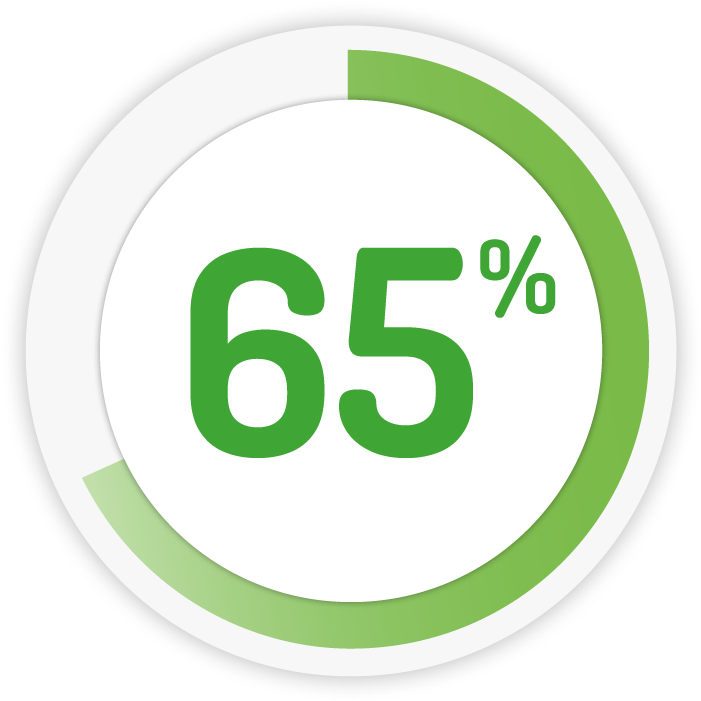

4g daily for 8 weeks

1g OD for 4 weeks
Achieving remission* in 2 weeks 9
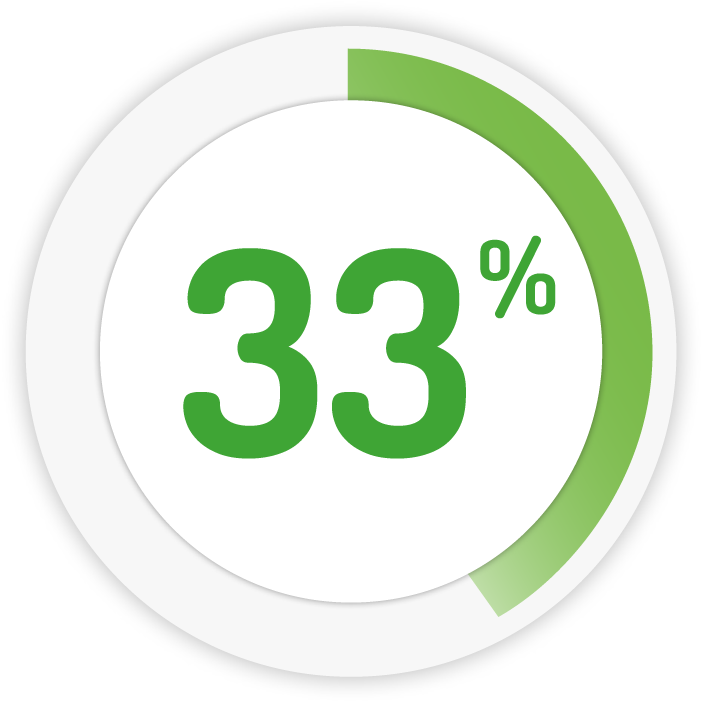

4g daily for 8 weeks

1g OD for 4 weeks
* Improvement: Patients (%) achieving a decrease in abbreviated UC-DAI of ≥ 2 from baseline
* Remission: Patients (%) achieving abbreviated UC-DAI < 2
PATIENT EXPERIENCE
In this video you will hear Amy’s UC journey from initial diagnosis to eventual treatment with PENTASA® sachets
RESOURCES
Visit our Resource Centre to see the range of support materials available for your patients
References
1. Pentasa Slow Release Tablets 500 mg. SmPC.
2. Pentasa Slow Release Tablets 1 g. SmPC.
3. Pentasa Sachet 1 g. SmPC.
4. Pentasa Sachet 2 g. SmPC.
5. Pentasa Sachet 4 g. SmPC.
6. Pentasa Enema 1 g / 100 ml. SmPC.
7. Pentasa Suppositories 1 g. SmPC.
8. Dignass AU, et al. Clin. Gastroenterol Hepatol. 2009;7(7):762–9.
9. Probert CSJ, et al. J Crohn’s Colitis. 2014;8:200–207.
10. Bokemeyer B, et al. J Crohn’s Colitis. 2012;6:476-482.
11. Flourie B, et al. Aliment Pharmacol Ther. 2013;37(8):767–775.
12. Iacucci M, et al. Can J Gastroenterol. 2010;24(2):127–133.
13. Salofalk 250 mg Tablets. SmPC.
14. Salofalk 500 mg Gastro-resistant tablets. SmPC.
15. Salofalk 1 g Gastro-resistant tablets. SmPC.
16. Salofalk 500 mg Prolonged Release Granules. SmPC
17. Salofalk 1 g Prolonged Release Granules. SmPC.
18. Salofalk 1.5 g Prolonged Release Granules. SmPC.
19. Salofalk 3 g Prolonged Release Granules. SmPC.
20. Octasa 400 mg Modified-release tablets. SmPC.
21. Octasa 800 mg Modified-release tablets. SmPC.
22. Octasa 1.6 g Modified-release tablets. SmPC.
23. Asacol 800 mg MR Tablets. SmPC.
24. Mezavant XL® 1.2 g Gastro-resistant, Prolonged Release Tablets. SmPC.
25. Salcrozine 500 mg Gastro-resistant Tablets. SmPC.
26. Salcrozine 1 g Gastro-resistant Tablets. SmPC.
27. Zintasa 400 mg Tablets. SmPC.
28. Fallingborg J, et al. Digest Dis Sci. 1993;38(11):1989-1993.
29. Layer PH, et al. Gastroenterology. 1995;108:1427-1433.
30. Lynch WD and Hsu R. Ulcerative Colitis. In: StatPearls. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459282/. Accessed: March 2025.
31. Lamb CA, et al. Gut. 2019;68(Suppl 3):s1-s106.





