
PENTASA sachets are indicated for use with yoghurt.3-5
Click on one of the links below
PENTASA HAS A RANGE OF FORMULATIONS TO OFFER PAEDIATRIC PATIENTS 1-5
PENTASA® has a range of oral formulations to suit your paediatric patients’ needs.
The following oral PENTASA® formulations are licensed to be taken in divided doses for achieving and maintaining remission in children from the age of 6 and upwards. 1-5
ORAL FORMULATIONS
4g sachet
2g sachet
1g sachet
1g tablet

500mg tablet
PENTASA OFFERS FLEXIBLE ADMINISTRATION WHICH CAN AID COMPLIANCE
TO AID WITH SWALLOWING
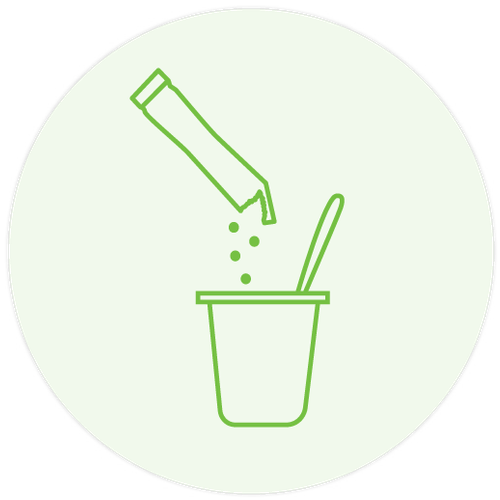
Sachets can be incorporated into a yoghurt pot or drink, or washed down with water or orange juice 3-5
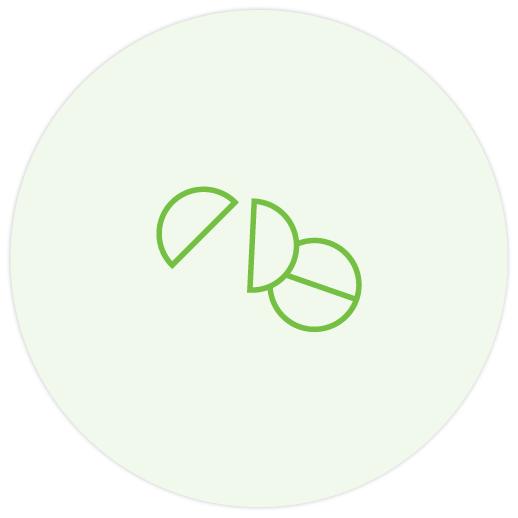
The 500 mg tablet can be broken, however it must not be crushed or chewed 1
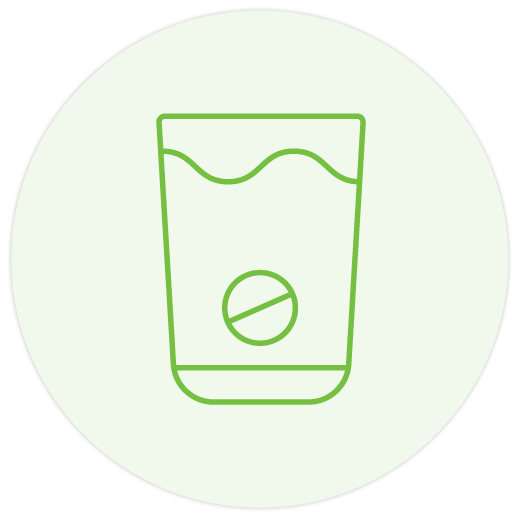
500 mg and 1 g tablets can be dispersed in 50 ml of cold water 1-2
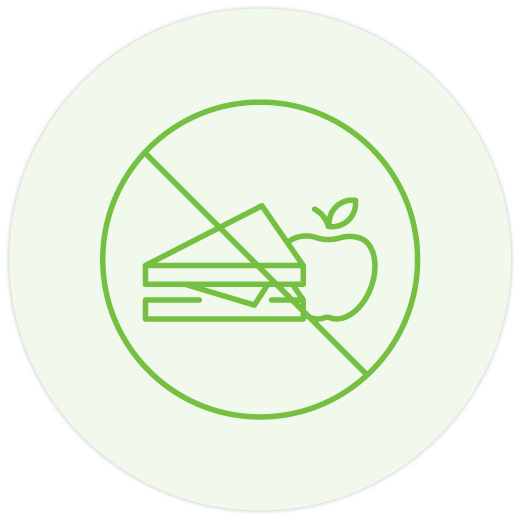
Pentasa can be taken with or without food 1-5
TWICE DAILY (BD) PENTASA DOSING IS EFFICACIOUS IN PAEDIATRICS 6
Primary endpoint was PUCAI score between the two arms (OD and BD dosing) at week 6. At week 6, there was no difference in median PUCAI score between the OD and BD groups (p=0.48). ∆
Complete remission* at week 6 6
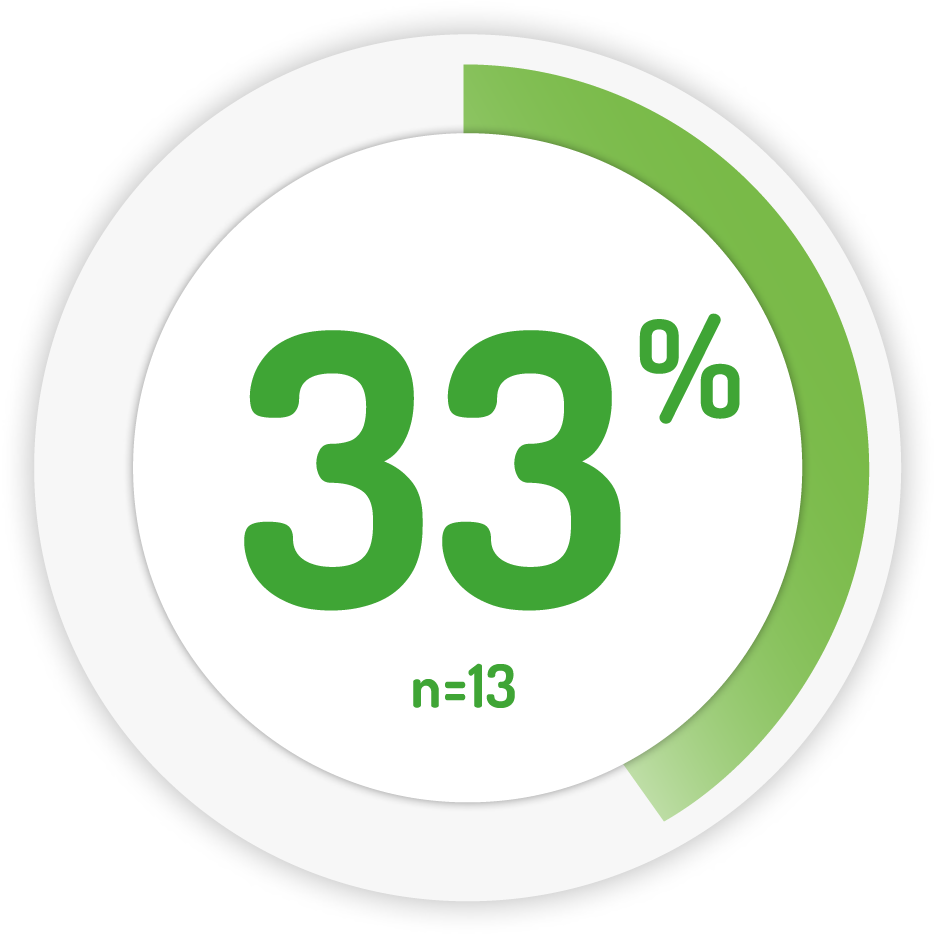

1g BD for 6 weeks †
Response** to treatment at week 6 6
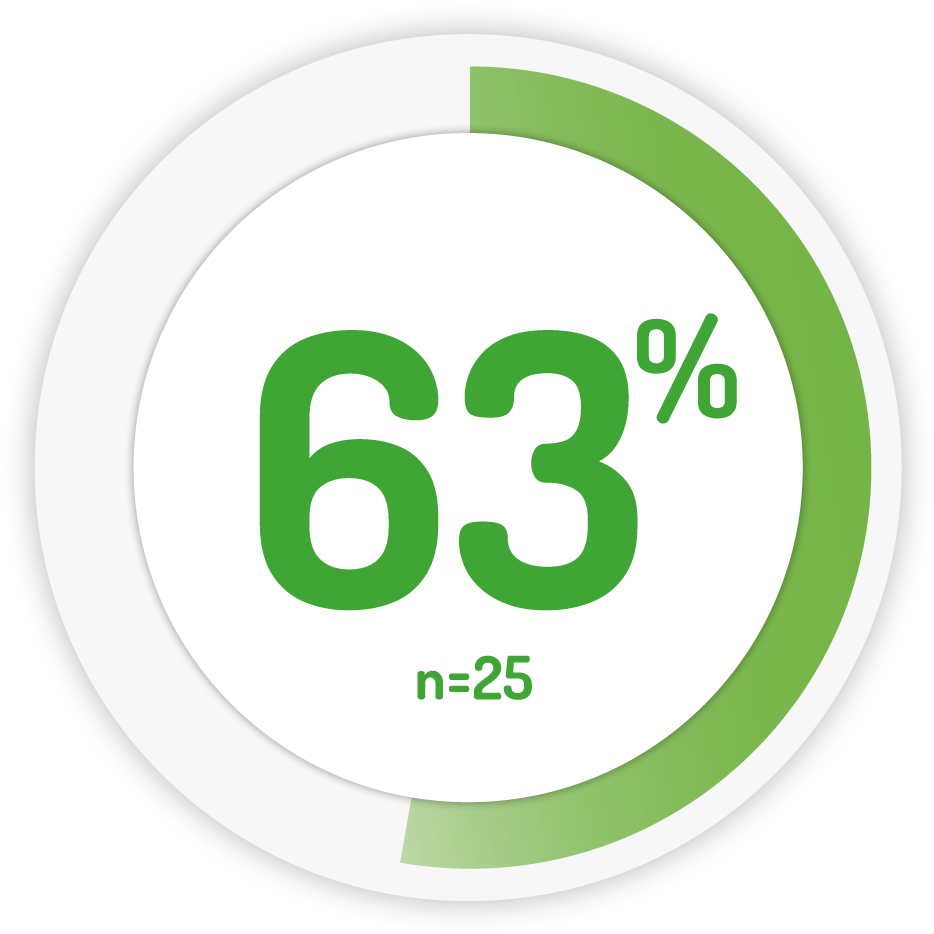

1g BD for 6 weeks †
∆ PENTASA is licensed to be taken in divided doses only in paediatrics. Weight based dosing based on 75 mg/kg/day
*COMPLETE REMISSION: PUCAI <10 points and a change of at least 10 points from baseline. PUCAI = Paediatric ulcerative colitis activity index.
**RESPONSE: PUCAI ≥10 points (active disease) but an improvement of at least 20 points
Note: PENTASA OD is not licensed for paediatric use in the UK.
PAEDIATRIC PATIENTS ARE COMPLIANT TO PENTASA TWICE-DAILY SACHETS 6
Compliance to twice-daily therapy at week 6
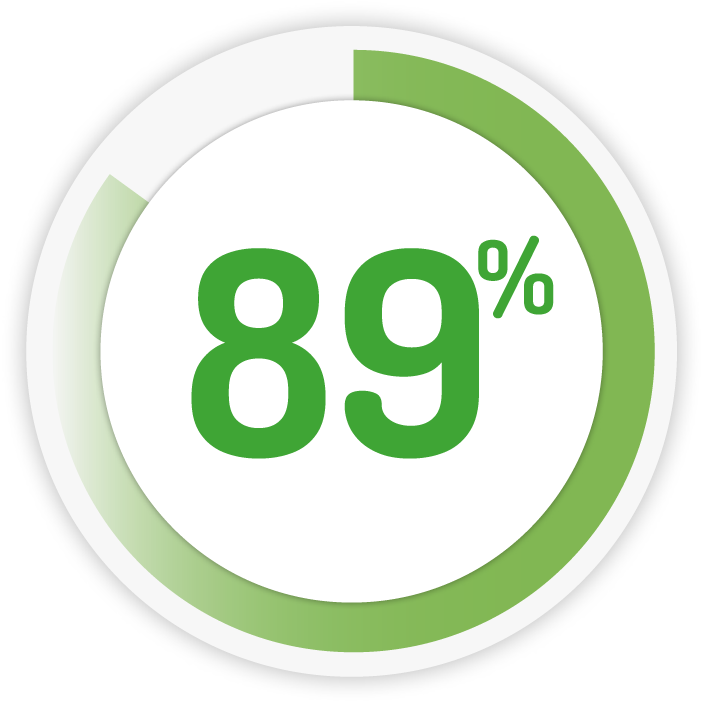

1g BD for 6 weeks
Granule-based formulations may reduce pill burden and encourage patient adherence. 7
References
1. Pentasa Slow Release Tablets 500 mg. SmPC.
2. Pentasa Slow Release Tablets 1 g. SmPC.
3. Pentasa Sachet 1 g. SmPC.
4. Pentasa Sachet 2 g. SmPC.
5. Pentasa Sachet 4 g. SmPC.
6. Turner D, et al. J Crohn’s Colitis. 2017;11:527–533.
7. Ye B and van Langenberg DR. World J Gastrointest Pharmacol Ther. 2015;6(4):137-144.




