

Adverse Event reporting information can be found in footer
Request a Meeting
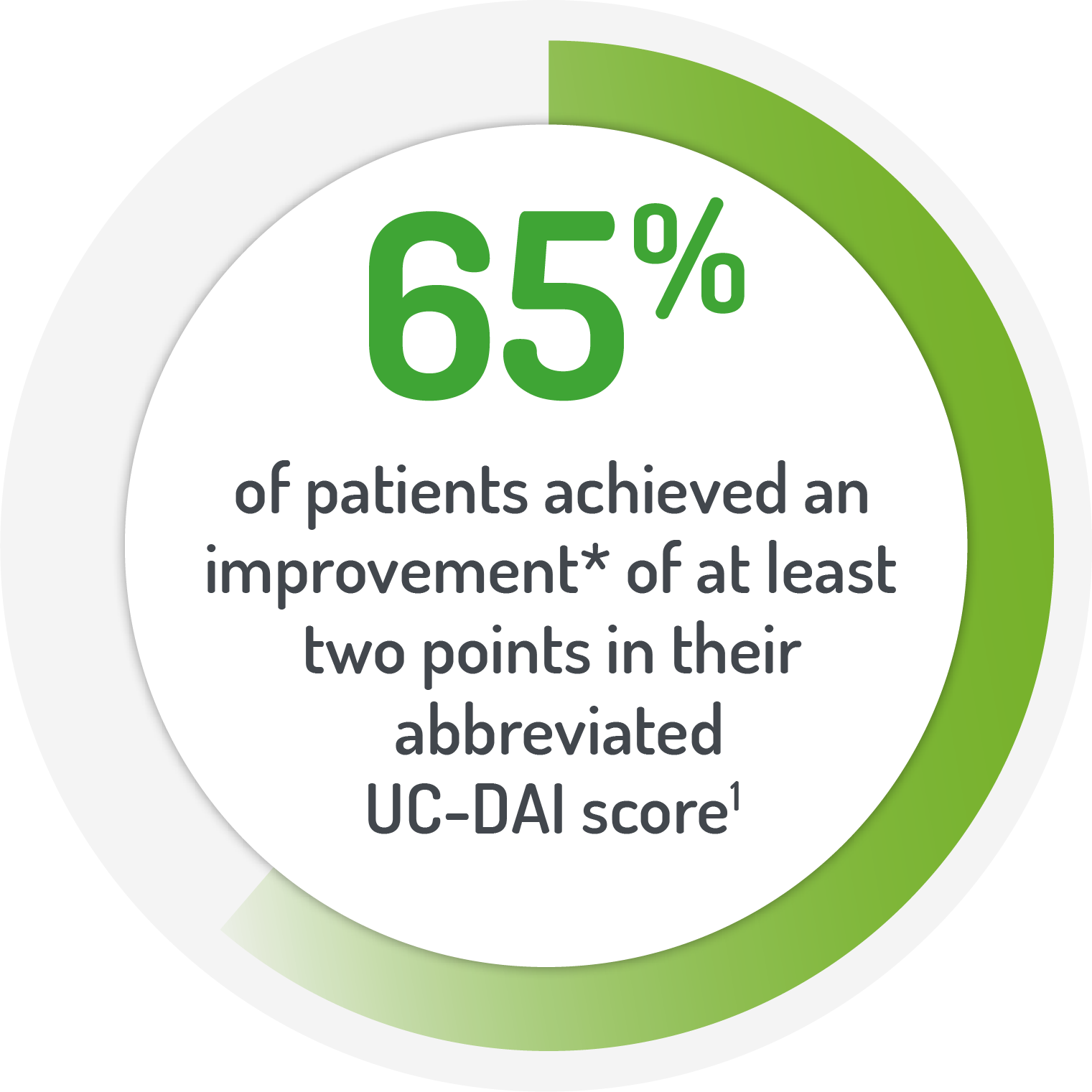
*Patients (%) achieving
a decrease in abbreviated
UC-DAI of >2 from baseline
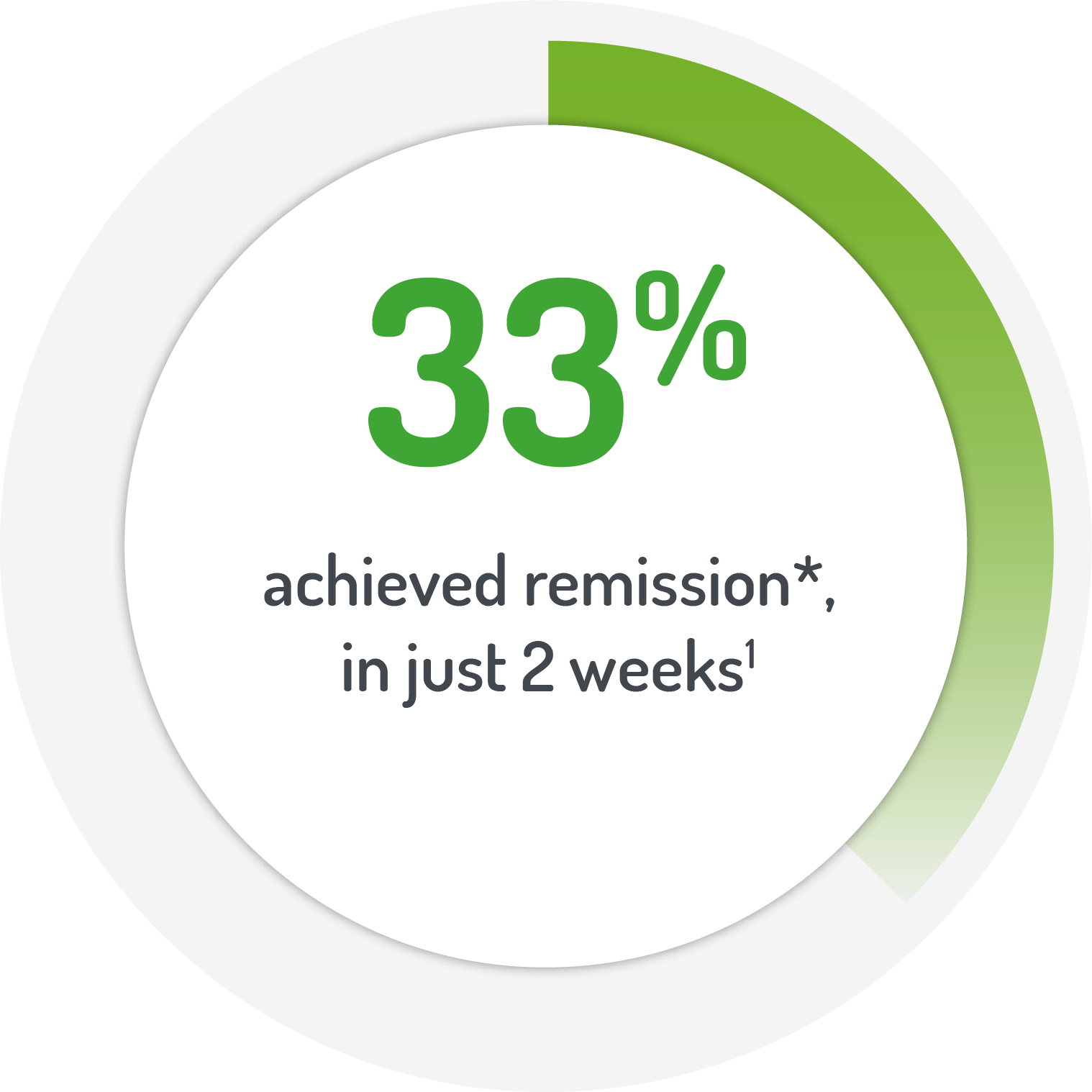
*Patients (%)
achieving abbreviated
UC-DAI <2
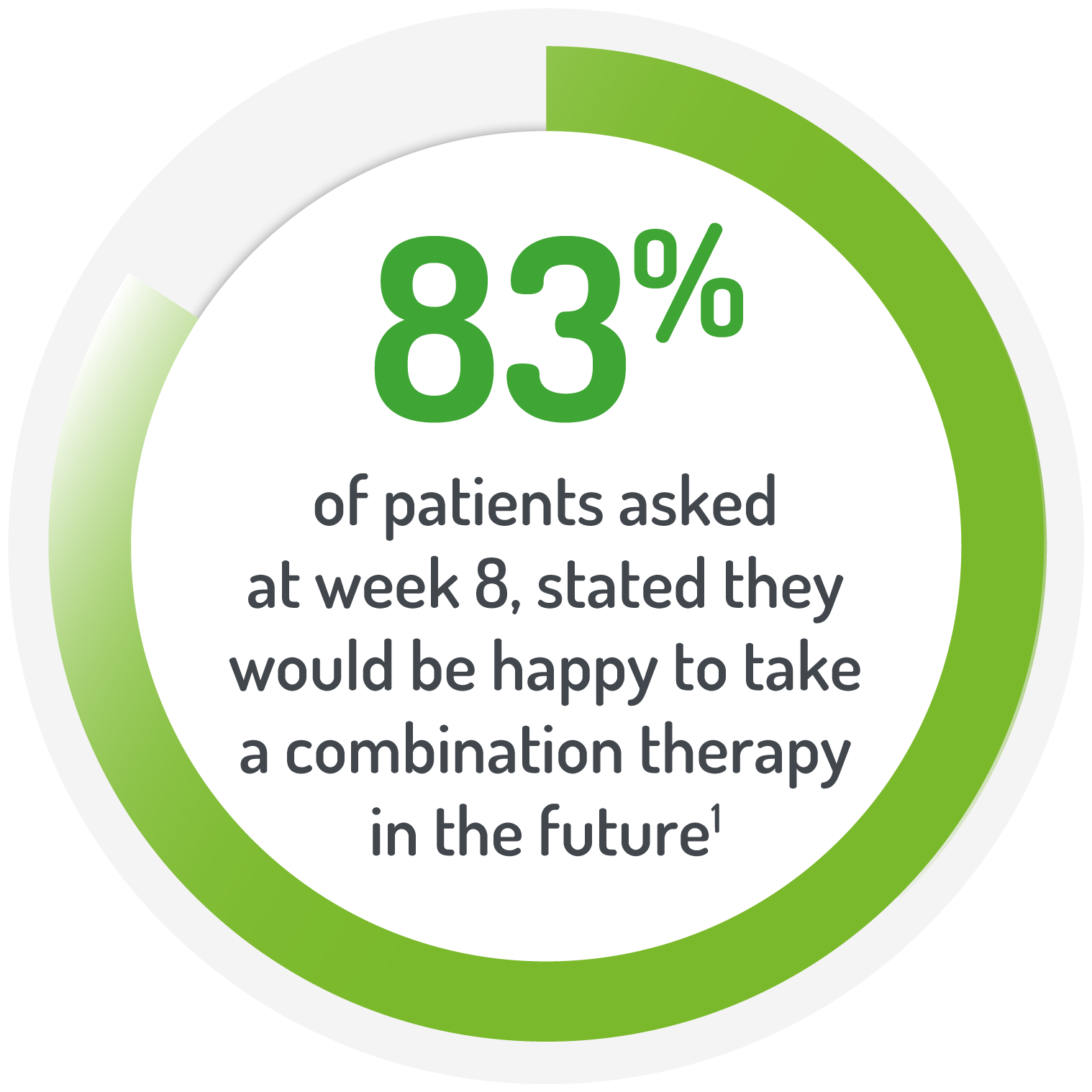
2g PENTASA sachet BD for 8 weeks +1g PENTASA enema (100 ml) for the 1st 4 weeks
STUDY DESIGN
Study design: 2 g PENTASA sachet BD + 1 g PENTASA® enema (100ml)
| Patient Numbers | Dose amount | Dose frequency | Rectal dose |
|---|---|---|---|
| 63 | 2g PENTASA | Twice-daily | 1g PENTASA enema for first 4 weeks |
| 53 | 2g PENTASA | Twice-daily | 1g placebo enema for first 4 weeks |
KEY INCLUSION CRITERIA
KEY EXCLUSION CRITERIA
SAFETY RESULTS FROM THE PINCE STUDY:
BD, Twice-daily; ITT, Intention-to-treat; NSAIDs, Non-steroidal inflammatory drugs; UC-DAI, Ulcerative colitis-disease activity index.
Job Code: UK-PA-2400044 - Date of preparation: November 2024