Adverse Event reporting information can be found in footer
Request a Meeting

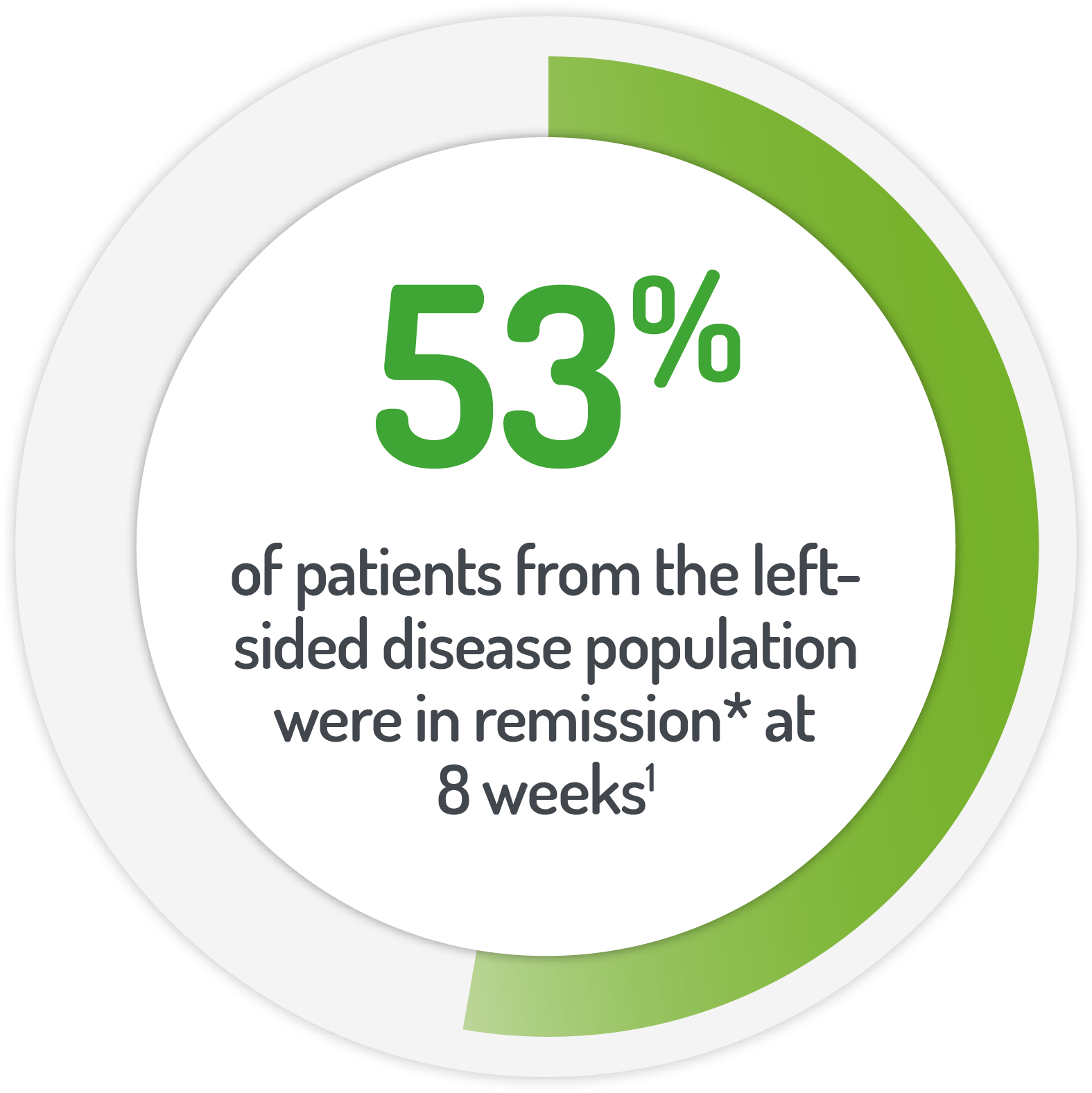
*Patients (%) achieving UC-DAI <1
The MOTUS study used 2 x 2 g PENTASA® sachets OD + 1 g enema (100ml) OD for the first four weeks
STUDY DESIGN
Results from the Phase IIIb, randomised, controlled, investigator blinded, non-inferiority, 8 week MOTUS study
| Patient Numbers | Dose amount | Dose frequency | Rectal dose |
|---|---|---|---|
| 102 (83 left-sided) | 2g PENTASA | Once-daily | 1g PENTASA enema for first 4 weeks |
| 104 (88 left-sided) | 4g PENTASA | Twice-daily | 1g PENTASA enema for first 4 weeks |
KEY INCLUSION CRITERIA
KEY INCLUSION CRITERIA
SAFETY RESULTS FROM THE MOTUS STUDY
| OD n=102 n (%) |
BD n=100 n (%) |
Total n=202 n (%) |
|
|---|---|---|---|
| Any TEAE | 33 (32.4) | 34 (34.0) | 67 (33.2) |
| TEAE experienced by >2% of patients | |||
| Abdominal pain | 3 (2.9) | 4 (4.0) | 7 (3.5) |
| Nausea | 5 (4.9) | 2 (2.0) | 7 (3.5) |
| Headache | 3 (2.9) | 3 (3.0) | 6 (3.0) |
| Worsening UC | 2 (2.0) | 3 (3.0) | 5 (2.5) |
| Pyrexia | 3 (2.9) | 2 (2.0) | 5 (2.5) |
| Proctalgia | 4 (3.9) | 0 (0.0) | 4 (2.0) |
| Arthralgia | 1 (1.0) | 3 (3.0) | 4 (2.0) |
| Asthenia | 1 (1.0) | 3 (3.0) | 4 (2.0) |
AE = adverse event, BD = twice-daily, OD = once-daily, TEAE = treatment emergent adverse event
Job Code: UK-PA-2400044 - Date of preparation: November 2024