

Adverse Event reporting information can be found in footer
Request a Meeting

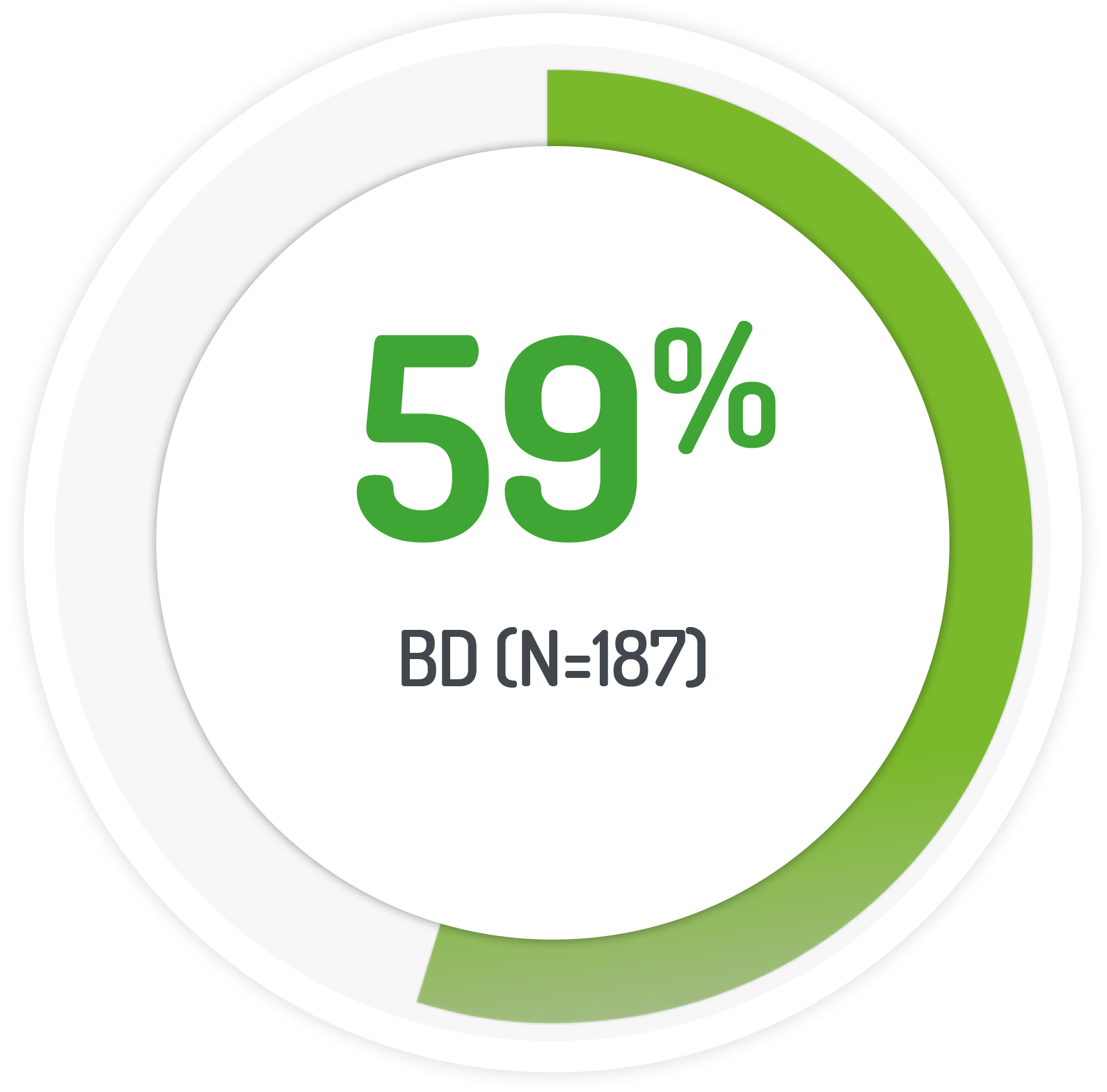
Kaplan-Meier estimated UC-DAI remission rate
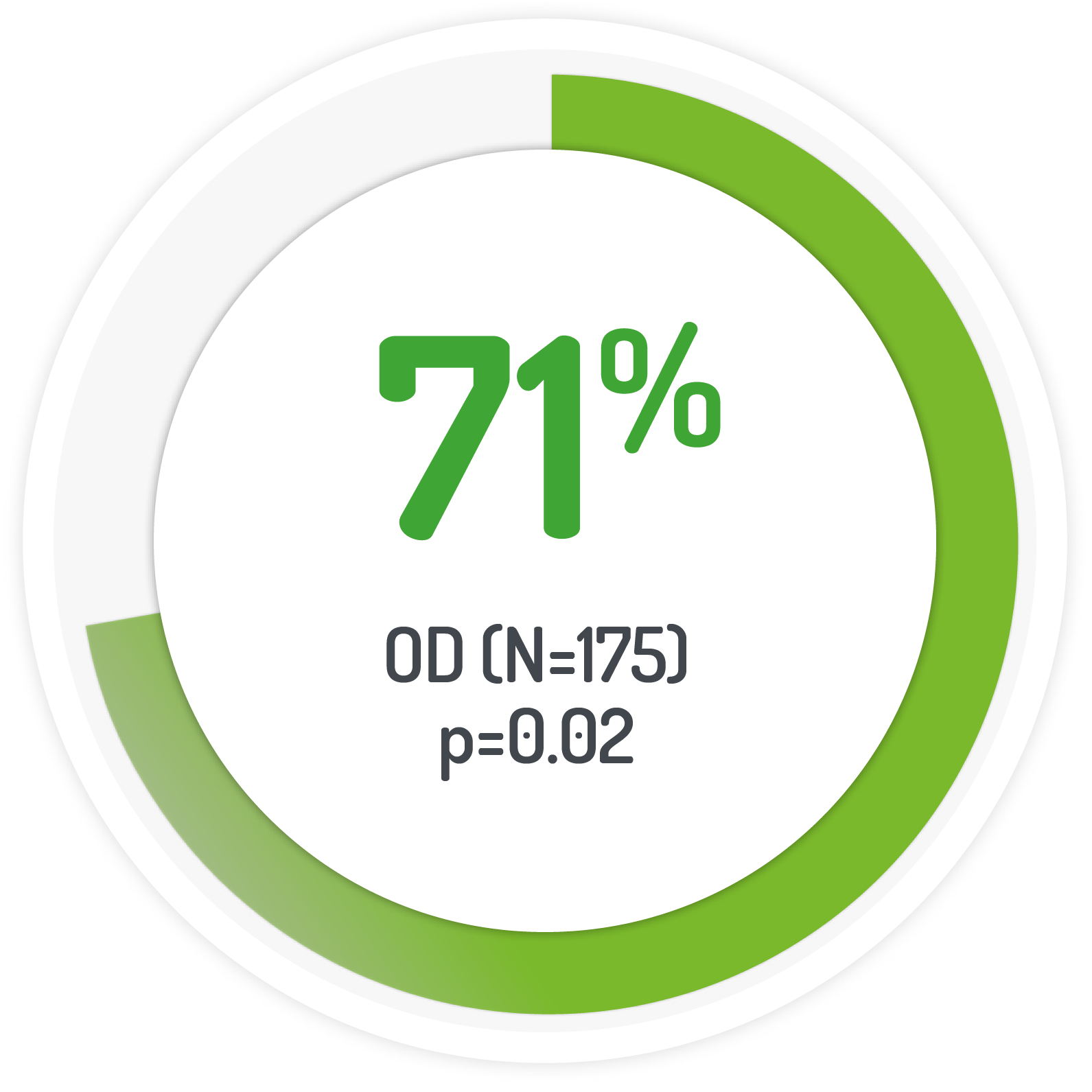
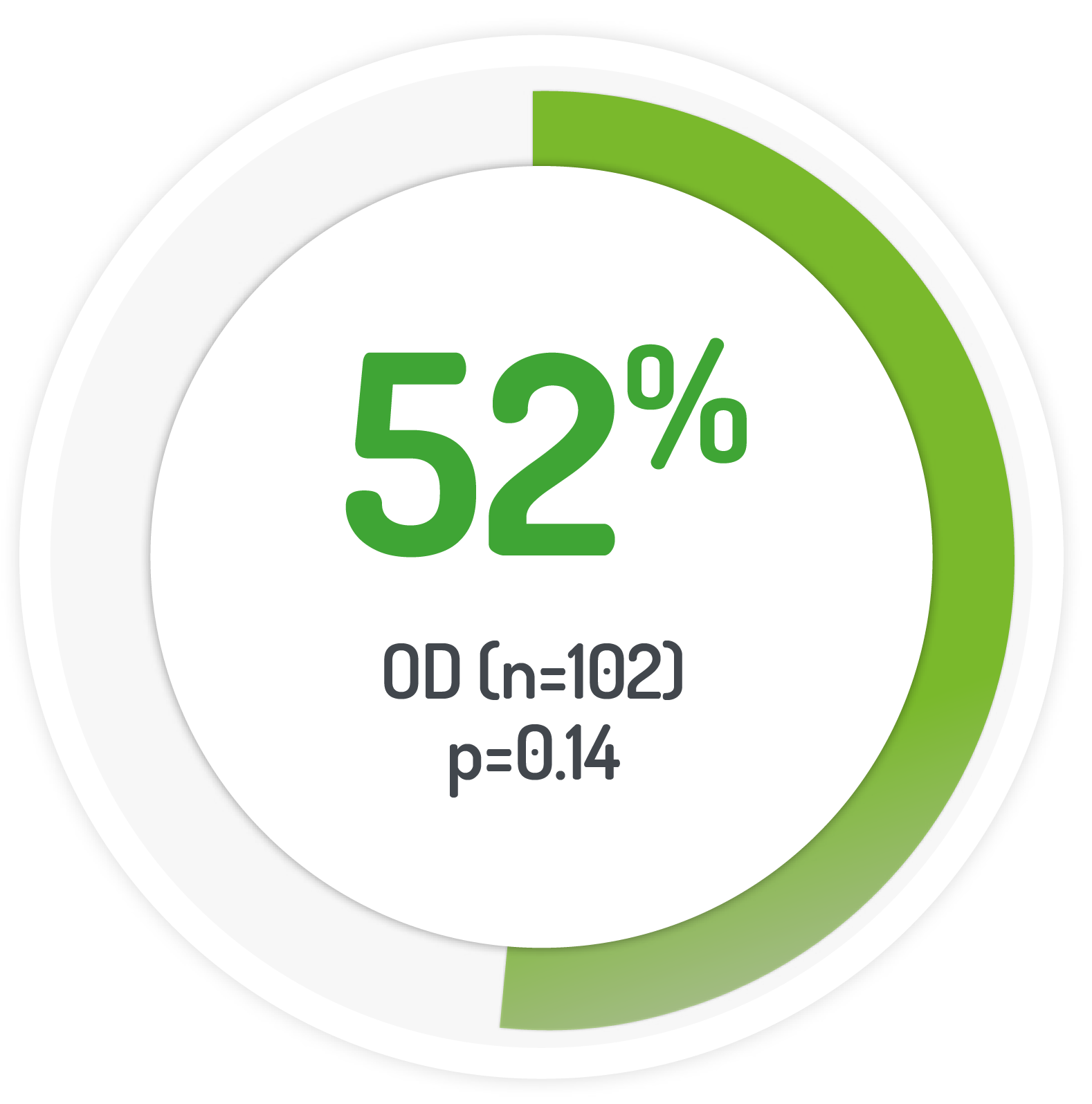
The PODIUM study used 2 g PENTASA sachet OD for 12 months
patients(%) achieving UC-DAI score s1
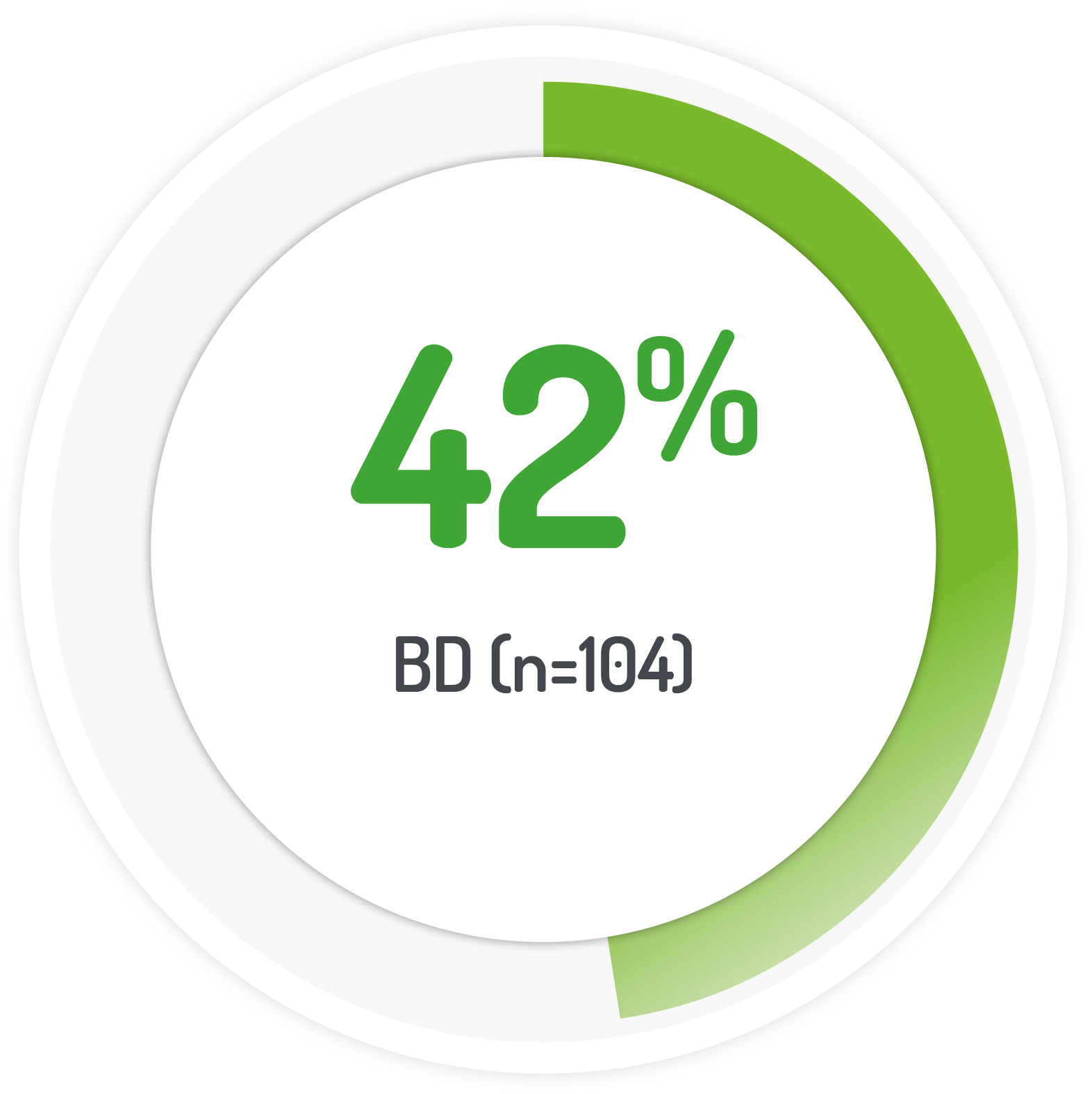
2g PENTASA sachet BD for 8 weeks +1g PENTASA enema (100 ml) for the 1st 4 weeks
normal stool frequency and cessation of bleeding


STUDY DESIGN
Results from the multicentre, randomised, single-blind, non-inferiority, 12 month PODIUM study
| Patient Numbers | Dose amount | Dose frequency |
|---|---|---|
| 175 (131 left-sided) | 2g PENTASA | Once-daily |
| 187 (128 left-sided) | 1g PENTASA | Twice-daily |
KEY INCLUSION AREA
KEY EXCLUSION AREA
SAFETY RESULTS FROM MOTUS STUDY
| Adverse events | 2 g mesalazine OD n=175 n (%) |
1 g mesalazine n=187 n (%) |
|---|---|---|
| Gastrointestinal disorder | 35 (20.0) | 24 (12.8) |
| Abdominal pain | 6 (3.4) | 5 (2.7) |
| Abdominal pain upper | 4 (2.3) | 3 (1.6) |
| Diarrhoea | 5 (2.9) | 4 (2.1) |
| Flatulence | 3 (1.7) | 4 (2.1)) |
| General disorders and administration site conditions | 7 (4.0) | 25 (2.7) |
| Infections and infestations | 30 (17.1) | 25 (13.4) |
| Bronchitis | 2 (1.1) | 5 (2.7) |
| Gastroenteritis | 4 (2.3) | 2 (1.1) |
| Nasopharyngitis | 10 (5.7) | 6 (3.2) |
| Sinusitis | 1 (0.6) | 4 (2.1) |
| Nervous system disorders | 5 (2.9) | 6 (3.2) |
| Skin/subcutaneous tissue disorders | 8 (4.6) | 2 (1.1) |
AEs = adverse events, BD = twice-daily, OD = once-daily.
Job Code: UK-PA-2400044 - Date of preparation: November 2024