

Adverse Event reporting information can be found in footer
Request a Meeting
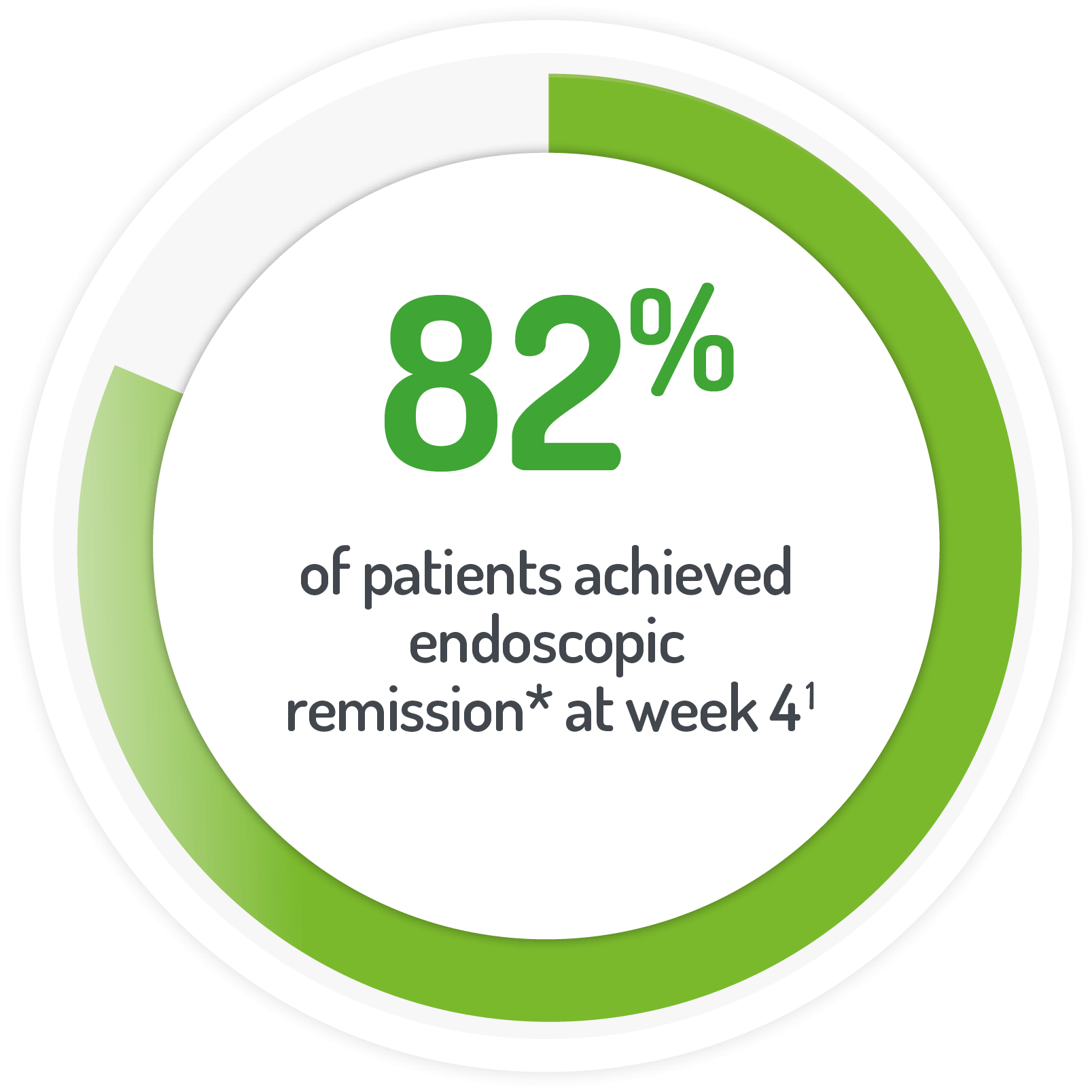
*Rectal mucosal score of 0 or 1 after 4 weeks of active treatment**
(or at time of discontinuation)
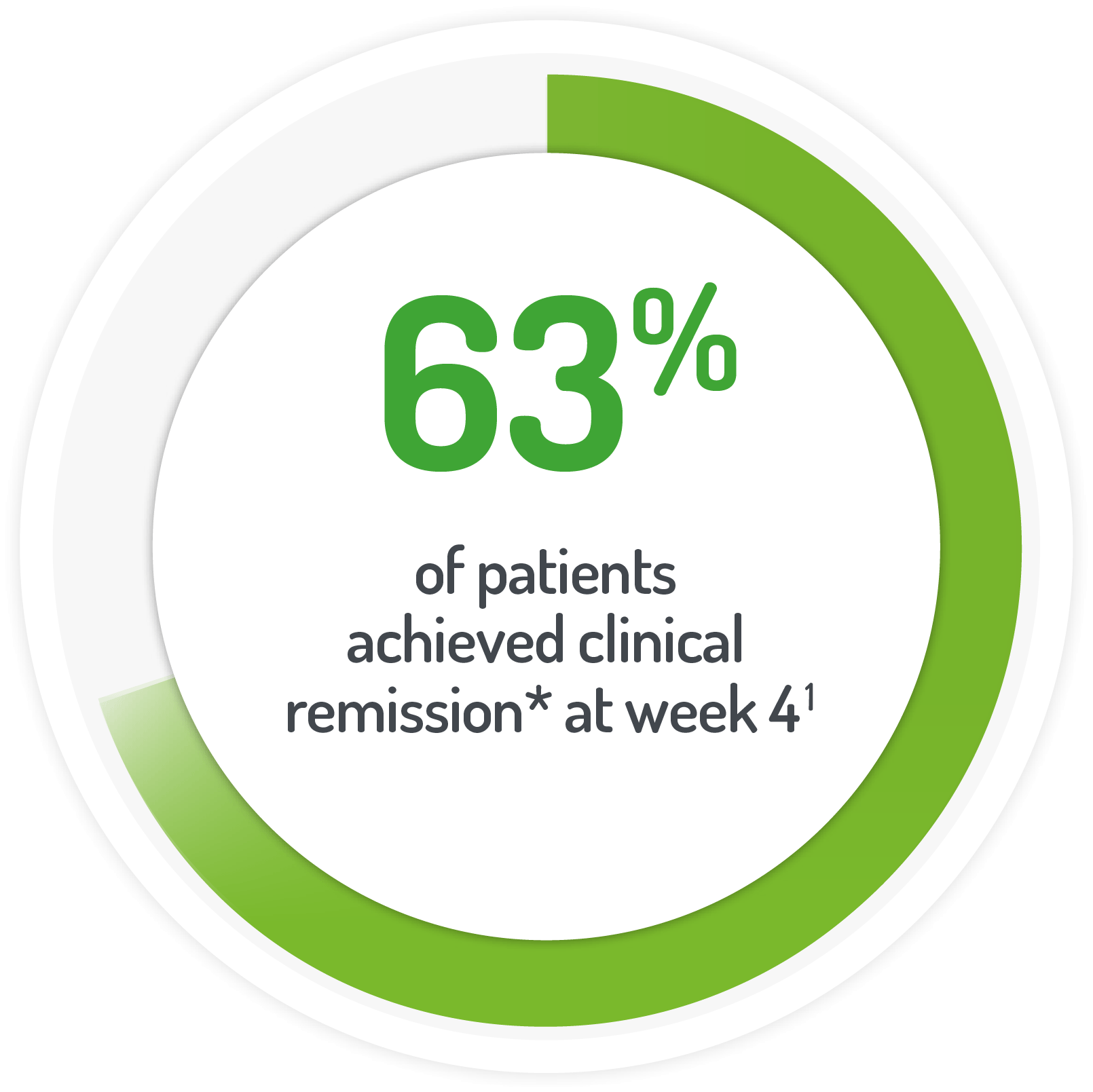
*UC-DAI score of 2
and rectal bleeding score of 0 after 4 weeks of active treatment**
(or at time of discontinuation)
N.B. Not all patients’ active treatment was monotherapy.
**68% of patients continued to receive standard dose mesalazine (≤2.4 g/day).
1 g PENTASA suppository OD for 4 weeks
STUDY DESIGN
Results from the phase III, multi-centre, randomised, double-blind, placebo-controlled, parallel group study (Watanabe et al. 2013) 1
| Patient Numbers | Dose amount | Dose frequency |
|---|---|---|
| 65 | 1g PENTASA | OD |
| 64 | 1g placebo | OD |
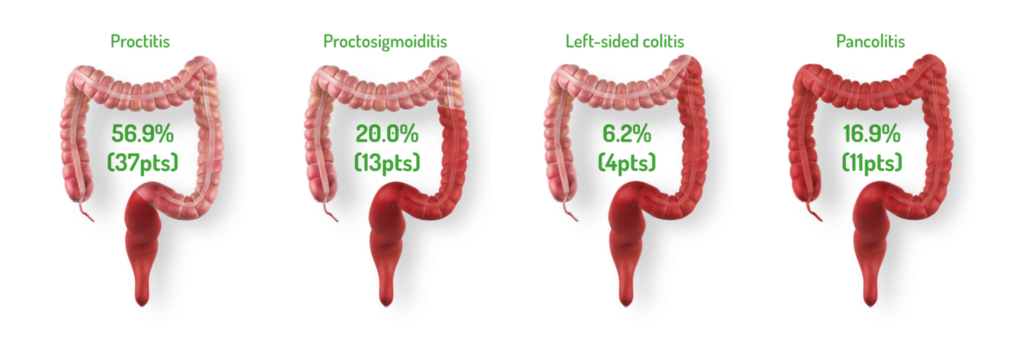
DISTRIBUTION AND EXTENT OF UC:
KEY INCLUSION AND EXCLUSION CRITERIA
IBD = Inflammatory bowel disease
SAFETY RESULTS FROM THE STUDY:
STUDY DESIGN
Results from a 4-week randomised, single centre investigator-blind study (Gionchetti et al. 1997) 3
| Mesalazine | Patient numbers | Dose amount | Dose frequency |
|---|---|---|---|
| PENTASA | 25 | 1 g | OD |
| Salofalk | 25 | 500 g | BD |
DAI = Disease activity index, PGA = Physician’s global assessment.
Salofalk and Claversal are different brand names for the same medicine.
Key inclusion and exclusion criteria
SAFETY RESULTS
Job Code: UK-PA-2400044 - Date of preparation: November 2024