
High and sustained patient satisfaction1
With DEGARELIX FERRING administration.1
What do patients think about DEGARELIX FERRING3,4?
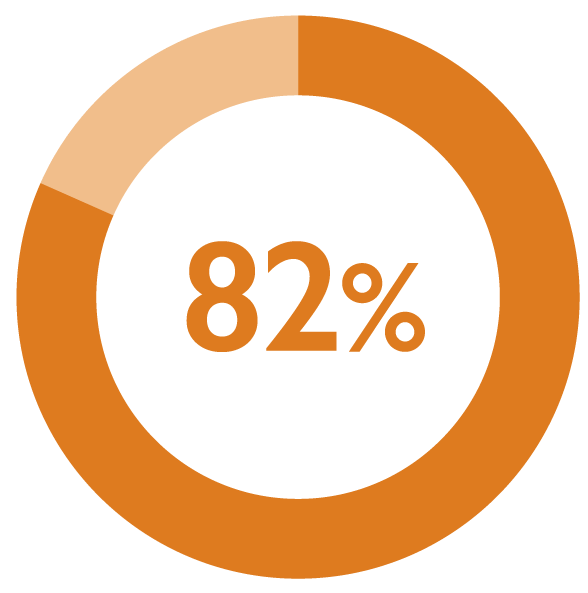
Monthly DEGARELIX FERRING administration was considered satisfactory by 82% of patients (173/211) at 6 months1*
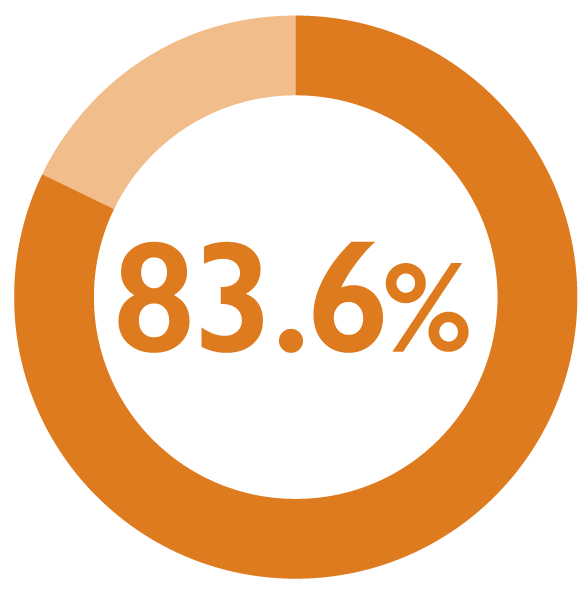
Satisfaction was sustained during the maintenance phase, with patient satisfaction rate increased to 83.6% (117/140) at 12 months1
*Patient satisfaction was the secondary endpoint in a multicentre, long-term, prospective, observational, non-interventional study of DEGARELIX FERRING patients in the Netherlands with advanced prostate cancer. The primary endpoint was PFS failure rate.
What are your key criteria for achieving a positive administration experience for patients?
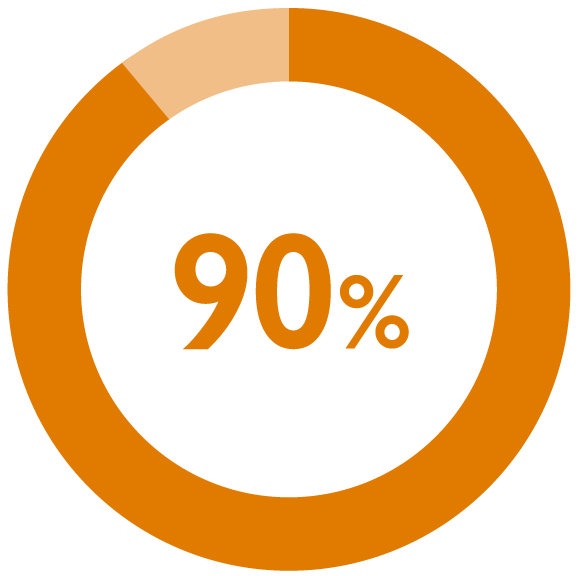
perceived seeing their urologist every 3 months as ‘just right’1
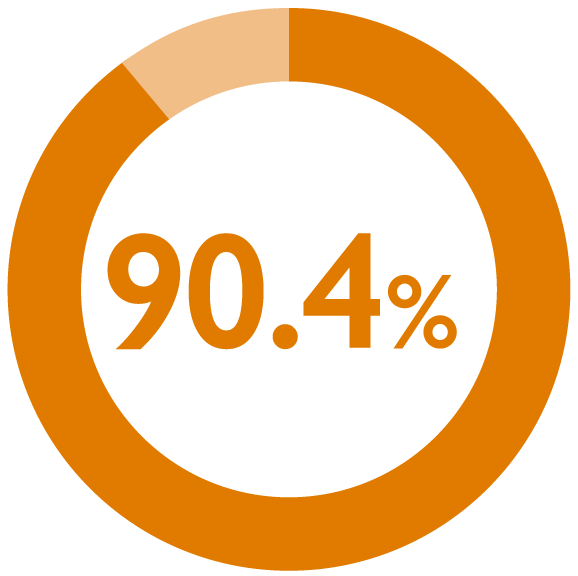
perceived monthly nurse visits for maintenance injections as ‘just right’1
*Patient satisfaction was the secondary endpoint in a multicentre, long-term, prospective, observational, non-interventional study of DEGARELIX FERRING patients in the Netherlands with advanced prostate cancer. The primary endpoint was PFS failure rate.
Secondary endpoints included patient and physician satisfaction scores.
Optimise your injection technique
Reconstitution and Administration Video Including Top Tips by Leanne McCourt
Summary of Top Tips from Leanne
References
1. Roshani H, et al. Curr Urol 2021;15:204–208.
2. Allchorne P, et al. Int J Urol Nurs 2022;16:127–137.
3. DEGARELIX FERRING 120 mg injection Summary of Product Characteristics. Ferring Pharmaceuticals Ltd. Available at: https://www.medicines.org.uk/emc/product/100906/smpc. Last accessed July 2025.
4. DEGARELIX FERRING 80 mg injection Summary of Product Characteristics. Ferring Pharmaceuticals Ltd. Available at: https://www.medicines.org.uk/emc/product/100905/smpc. Last accessed: July 2025.
5. Klotz L, et al. BJU Int 2008;102:1531-1538.


